Radiopharmacies Market Forecast 2026–2036: Market to Reach USD 24.8 Billion by 2036 at 7.1% CAGR
NY, DE, UNITED STATES, February 19, 2026 /EINPresswire.com/ --
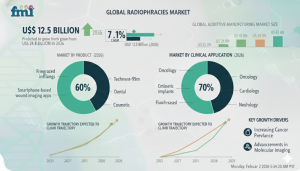
NEWARK, DE —The global radiopharmacies market is projected to grow from USD 12.5 billion in 2026 to USD 24.8 billion by 2036, expanding at a compound annual growth rate (CAGR) of 7.1%. This steady expansion reflects sustained increases in PET procedure volumes, broader clinical adoption of targeted radiotracers, and growing reliance on specialized radiopharmacy operations to ensure dose integrity within strict time constraints. As nuclear medicine workflows scale, centralized and compliant radiopharmacy models remain essential to support high-throughput diagnostic and theranostic applications.
Market snapshot: global market 2026 - 2036
Market size in 2026? USD 12.5 billion
Market size in 2036? USD 24.8 billion
CAGR (2026–2036)? 7.1%
Leading radiopharmacy type? PET radiopharmacy (largest share, dominant due to high procedural throughput in oncology, neurology, and cardiology)
Leading radiotracer class? 18F-FDG (34.0% of overall radiotracer demand)
Leading end user? Hospitals and imaging centers (jointly accounting for the majority of demand)
Key growth regions? United States (highest share and strong commercial networks), China (fastest expansion), South Korea, and major European markets
Top companies? Cardinal Health, Curium, Jubilant Radiopharma, Siemens Healthineers, Lantheus, GE HealthCare, Eckert & Ziegler, Bracco Imaging, SOFIE, IBA
Market Momentum (YoY Path)
The radiopharmacies market is expected to follow a consistent upward trajectory through the forecast period. Starting at USD 12.5 billion in 2026, the market demonstrates reliable annual progression supported by rising PET and SPECT volumes, theranostics-linked tracer demand, and centralized supply models. Key milestone values include continued compounding growth toward 2028, 2030, 2031, 2033, and ultimately reaching USD 24.8 billion by 2035–2036, driven by structural shifts toward compliance intensity, tracer diversification (beyond 18F-FDG to PSMA, neuro tracers, and DOTATATE/NET), and enhanced on-time delivery performance.
Why the Market is Growing
Demand for radiopharmacies continues to rise due to the time-sensitive nature of nuclear medicine workflows, where tracer availability, batch release discipline, and compliance are critical. Sustained PET procedure growth anchors demand in PET radiopharmacy, while SPECT maintains a durable role in established diagnostics. Commercial centralized models gain traction through scale efficiency, standardized cGMP practices, and hub-and-spoke distribution that balance geographic reach with tight timing constraints. Broader radiotracer portfolios—including PSMA, neuro (amyloid/tau), and DOTATATE/NET—are integrating into routine clinical ordering, further increasing operational requirements for reliable, compliant supply to hospitals and imaging centers.
Segment Spotlight
Radiopharmacy Type PET radiopharmacy secures market leadership with the largest share among radiopharmacy types. It is driven by high throughput intensity, time-sensitive dispatch needs, daily routine cycles, and strong utilization in oncology, cardiology, and neurology. Centralized infrastructure investments—cyclotrons, cleanrooms, and skilled radiochemist teams—support scalable, multi-site servicing and reinforce PET radiopharmacy as the primary growth engine in the radiopharmacies market.
Radiotracer Class 18F-FDG remains the leading radiotracer class, holding 34.0% of overall demand handled through radiopharmacy operations. As the backbone for routine PET imaging, it delivers stable, predictable production volumes and scheduling. Its standardized synthesis, quality control, and distribution processes within short half-life constraints enable repeatable high-throughput operations, anchoring capacity planning across hospitals and imaging centers in the radiopharmacies market.
Drivers, Opportunities, Trends, Challenges
Drivers Key growth is propelled by rising PET and SPECT procedure volumes, increasing theranostics-linked radiotracer demand, and stricter delivery-time requirements that favor local production and distribution networks. Compliance-driven models, including tightening cGMP standards and centralized scaling, reinforce institutional reliance on external radiopharmacy supply.
Opportunities Tracer portfolio expansion (PSMA, neuro tracers, DOTATATE/NET) alongside PET infrastructure build-out creates opportunities for flexible synthesis, modular production, and digital tools that improve scheduling, dose forecasting, and traceability. Centralized cGMP operations and hub-and-spoke networks offer scale advantages for providers serving multi-site imaging ecosystems.
Trends The market is shifting toward commercial centralized and hub-and-spoke service models, automation in daily production cycles, and digital orchestration for reduced wastage and on-time performance. Advanced radiotracers are increasing the need for tracer-specific QC and controlled handling, while regulatory rigor emphasizes audit-ready documentation and radiation safety.
Challenges Short half-life constraints demand precise timing and minimize batch variability, raising complexity in scheduling and regulatory documentation. Balancing compliance intensity with expanding tracer diversity requires sustained investment in validated processes, cleanroom infrastructure, and chain-of-custody controls.
Competitive Landscape
Competition in the radiopharmacies market centers on regulatory handling expertise, network density, radiotracer portfolio breadth, and reliable time-sensitive supply. Leading players such as Cardinal Health and Curium leverage distribution footprints, operational standardization, and centralized models to differentiate. Strategic focus includes PET infrastructure adjacency to imaging routes, scaling hub-and-spoke networks, and capabilities across cGMP commercial, hospital nuclear, and clinical trial models. Tracer diversification and compliance discipline determine positioning as demand grows through 2036.
Get Access of Report Sample: https://www.futuremarketinsights.com/reports/sample/rep-gb-32081
Scope of the Report
Quantitative Units: USD billion (market valued at USD 24.8 billion by 2036)
Segmentation: Radiopharmacy Type, Service Model, Radiotracer Class, Regulatory Handling, End User
Regions Covered: North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Countries Covered: USA, Germany, UK, France, Japan, China, South Korea, and 40+ additional countries
Explore More Related Studies Published by FMI Research:
Demand for Medical Bionic Implant and Artificial Organs in Japan: https://www.futuremarketinsights.com/reports/japan-medical-bionic-implant-and-artificial-organs-market
Electrophysiology Market: https://www.futuremarketinsights.com/reports/electrophysiology-market
Triple Therapy Inhalers Market: https://www.futuremarketinsights.com/reports/triple-therapy-inhalers-market
Disease-Modifying MS Therapies Market: https://www.futuremarketinsights.com/reports/disease-modifying-ms-therapies-market
Vitamins and Minerals Based Injectables Market: https://www.futuremarketinsights.com/reports/vitamins-and-minerals-based-injectables-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Why FMI: Decisions that Change Outcomes- https://www.futuremarketinsights.com/why-fmi
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.